Research Article Summary
We’ve been blocking immune checkpoint proteins for a decade. Is the next wave…glycans??
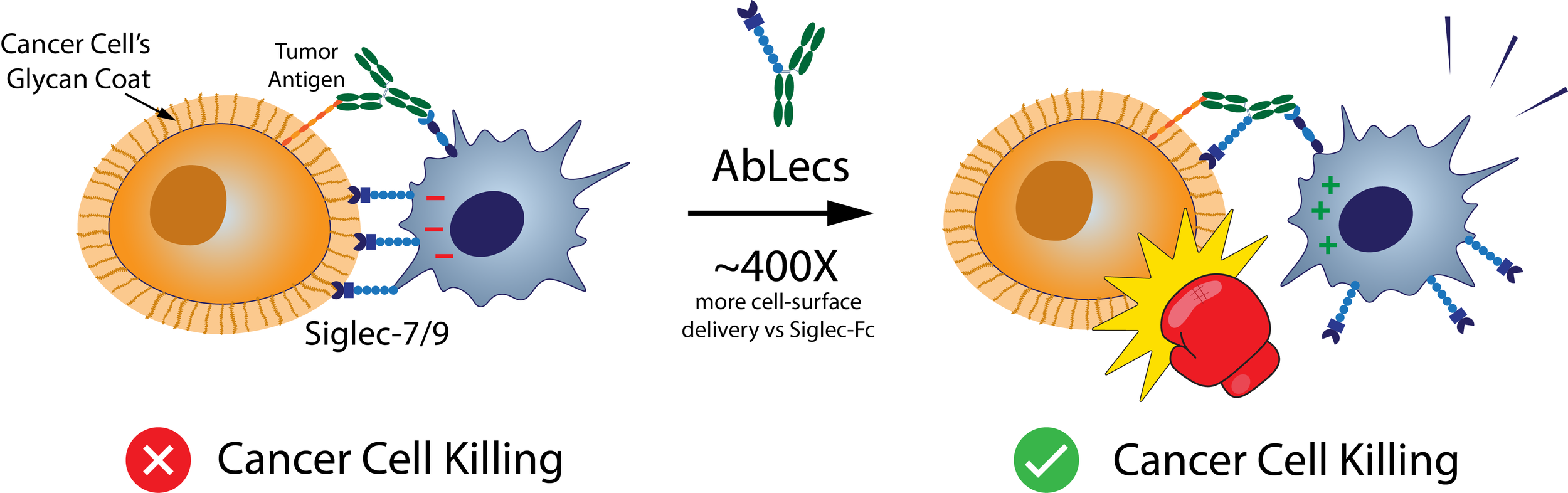
The recent Nature Biotechnology paper from Stark et al goes after glyco-immune checkpoints, the sugar coating on cancer cells that helps them evade immune system detection. Many tumors overexpress sialylated glycans on their cell surface which bind inhibitory receptors like Siglec-7 and Siglec-9 on immune cells to dampen the immune response.
We’ve known about cancer’s sugar-coded “don’t eat me signals” for years now, but previous attempts to target them have failed because glycans are notoriously hard to drug. They are expressed all over the body and are typically bound with low affinity. Therefore, most attempts to target them have suffered because they fail to achieve good enough specificity and a high enough local concentration at the target site where immune killing happens.
Enter AbLecs (antibody-lectin chimeras) - a bispecific format where a high‑affinity antibody arm anchors onto a chosen cell-surface target, such as HER2, while the other arm is a lectin decoy receptor that soaks up immunosuppressive glycans right at the immune synapse.
AbLecs could be a game changer because they turn weak glycan interactions into something therapeutically viable.
They are ~400X more effective at delivering Siglec-7 binding domains to the cancer cell surface than traditional Siglec-7-Fc constructs.
They show strong phagocytosis and NK mediated cancer cell killing in primary human immune cell assays, often better than their parent antibodies.
Mechanistically, they seem to prevent Siglecs from accumulating at the immune synapse, thus removing the brake that cancer’s glycan coat presses.
They show early but encouraging in vivo activity in a humanized lung colonization model, with a metastasis reduction on par with, or slightly exceeding, the parent antibody.
The next challenges will be broader in vivo validation and pharmacokinetics.
They will need to expand in vivo validation across more models and endpoints, while also grappling with key mouse/human differences in sialic acid biology, particularly that mice produce Neu5Gc, a form of sialic acid that humans do not.
PK will be important. In this study, AbLecs were cleared on the order of hours to days whereas the parent antibody, Trastuzumab, was cleared in about two weeks.
Nevertheless, this technology has a lot of potential. Its modular design means it can be used to target several cancer cell targets (CD20, EGFR, HER2, etc.) and glyco-immune checkpoints (Siglec-7, Siglec-9, Galectin-9). I’m excited to see more from the labs of Jessica Stark, Carolyn Bertozzi, and from their company Valora Therapeutics which is advancing the AbLec platform for cancer immunotherapy!
Link to full article: https://www.nature.com/articles/s41587-025-02884-6#Abs1
Manuscript Introduction
The blood-brain barrier (BBB) describes a set of properties unique to the vasculature of the central nervous system (CNS) that tightly regulates the movement of ions, molecules, and cells between the blood and the brain1. The BBB is critical for maintaining brain homeostasis and its dysfunction is a hallmark of many neuroinflammatory diseases, including multiple sclerosis, epilepsy, and stroke2. One component of the BBB that remains poorly understood is the BBB glycocalyx, the dense glycan-rich meshwork that lines the luminal surface of the CNS vasculature2–4.
All blood vessels are lined with a glycocalyx that serves as the interface between the blood and vasculature, but the composition and properties of each glycocalyx are unique to a given vascular bed or location in the vascular tree5–7. The glycocalyx is composed of various glycoconjugates, particularly the long, linear, negatively charged glycans known as glycosaminoglycans (GAGs), which include heparan sulfate (HS), chondroitin sulfate (CS), and hyaluronan8–10. Most proteins on the luminal surface of endothelial cells (ECs) are glycosylated, with their asparagine-linked N-glycans typically capped by sialic acid, a negatively charged monosaccharide that provides much of the negative charge of the cell surface11,12. The glycocalyces of the peripheral vasculature (PV) sense blood flow, regulate blood clotting, and control the endothelial microenvironment9,13. Additionally, several PV glycocalyces limit vascular permeability by acting as a filtration barrier, in which their degradation increases vascular permeability14–16.
During inflammation, there are several proposed roles for the glycocalyx. Some studies suggest it acts as a physical barrier, whereupon its degradation in inflammation increases vascular permeability and allows leukocytes to interact with previously shielded adhesion molecules and cross the endothelium17,18. Conversely, the glycocalyx may also be pro-inflammatory, serving an integral role in leukocyte recruitment by enabling the formation of chemokine gradients and because several of its components, including HS and hyaluronan, serve as ligands that are essential for leukocyte interactions5,19,20. Therefore, the glycocalyx may have a complex role in modulating inflammation with both pro- and anti-inflammatory properties5.
While PV glycocalyces have been more thoroughly studied, the BBB glycocalyx is only recently gaining attention4,6,21–23. Recent investigations demonstrated that the BBB glycocalyx was significantly thicker than the heart and lung glycocalyces in capillaries, indicating that the BBB glycocalyx may have unique characteristics24. The BBB glycocalyx also prevented large but not small molecules from reaching the EC surface, thus serving as a molecular sieve, like in the PV, and acting as the first component in the BBB22. There is evidence for BBB glycocalyx degradation in neuroinflammatory conditions such as aging, multiple sclerosis, cardiac arrest, stroke, sepsis, and cerebral malaria23–30. Despite these promising discoveries, the BBB glycocalyx remains a highly enigmatic and poorly understood structure, with many fundamental questions still unanswered. Which glycans are most abundant in the BBB glycocalyx, and how does molecular composition vary between glycocalyces? Which glycans contribute most to BBB structure and function? How does the BBB glycocalyx vary across the vascular tree? How does neuroinflammation affect the BBB glycocalyx?
Here, we show that the BBB glycocalyx is highly distinct from PV glycocalyces. Improving upon previous electron microscopy (EM) methods, we show that (i) the BBB glycocalyx is significantly thicker than PV glycocalyces, (ii) its structure remains relatively uniform across the vascular tree, and (iii) the presence of hyaluronan is critical for its structure. Using a novel glycomics approach, we find that the BBB glycocalyx is enriched in sialic acid, CS, and hyaluronan compared with kidney and liver glycocalyces. Using endothelial RNA sequencing, we identify potential genetic determinants for these differences, including BBB enrichment of genes involved in sialic acid addition and peripheral enrichment of Tmem2 and Hyal2, the genes encoding the only known cell-surface hyaluronidases11. We find that glycocalyx degradation is not sufficient to alter BBB permeability in health, but that it regulates barrier permeability during BBB dysfunction, indicating that the primary barrier function of the BBB glycocalyx may be to regulate access to the CNS endothelium22. Contrary to previous studies, we show that neuroinflammation during the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis leaves the BBB glycocalyx largely unchanged, even at sites of active leukocyte extravasation. Moreover, genetic removal of CNS endothelial sialic acid causes delayed onset of paralysis in EAE. Taken together, these findings highlight the unique structural, molecular, and functional characteristics of the BBB glycocalyx in health and neuroinflammation.